Seychelles Becomes the Fourth Country that Approves BIBP’s COVID-19 Vaccine for Emergency Use
On February 25, 2021, the Ministry of Health (MOH) of Seychelles officially approved the Inactivated COVID-19 Vaccine (Vero Cell) developed by Beijing Institute of Biological Products Co., Ltd. (BIBP), China National Biotec Group Company Limited (CNBG), Sinopharm (BIBP’s COVID-19 Vaccine) for registration and marketing in Seychelles, making Seychelles the fourth overseas country that approved the BIBP’s COVID-19 vaccine for marketing following the UAE, Bahrain and Bolivia.
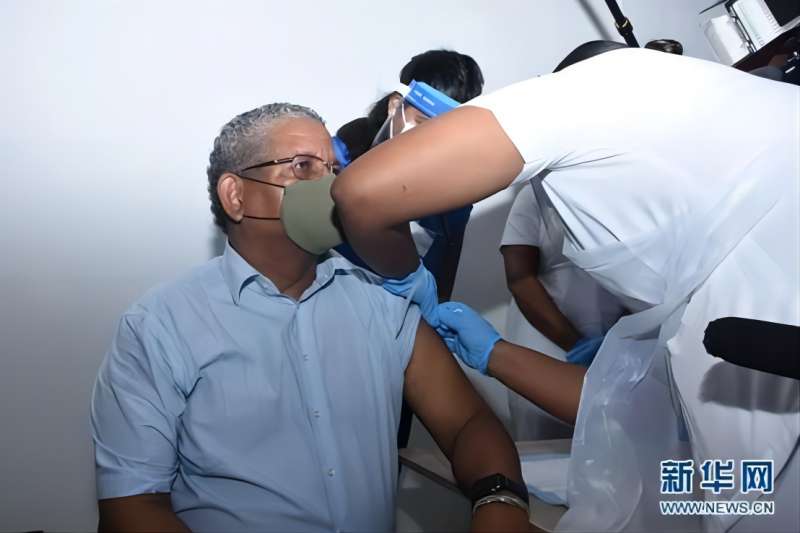
Earlier on January 10, Seychelles News Agency reported that President Ramkalawan was vaccinated with the Inactivated COVID-19 Vaccine (Vero Cell) developed by BIBP on the day, becoming the first head of state in Africa to get this vaccine. He also launched a mass vaccination campaign nationwide.
▲
On January 10, Seychelles President Ramkalawan Was Vaccinated with the COVID-19 Vaccine at an Vaccination Site in Vittoria, Capital of Seychelles. Photo from WWW.NEWS.CN.
On January 5, MOH Seychelles announced the start of the emergency use campaign of COVID-19 vaccine in Seychelles, with priority given to medical staff and those in essential positions. The vaccination campaign would gradually move from Mahe Island, where Victoria is located, to the rest of the islands, covering 70% of the population to achieve herd immunity. The BIBP’s vaccines were used during the first batch of vaccination
Seychelles officially put the COVID-19 vaccines into emergency use on January 10. President Ramkalawan was vaccinated with BIBP’s vaccine first, followed by Former President Danny Faure, Cabinet ministers and members of the National Assembly. The vaccination campaign was broadcast live by Seychelles TV.
Ramkalawan said in a speech after vaccination that he took the lead in vaccination to show the Seychelles people that the Chinese vaccine was safe, and he called on all Seychelles people to get this vaccine. Several members of the National Assembly said in the interviews after vaccination that they felt fine and that they would encourage others around them to get vaccination as soon as possible to achieve herd immunity.
Since January 11, medical staff and police in Seychelles have also started to get the Chinese vaccines. Gordion, Director-general of Public Health of Seychelles, said the vaccine had been tested well in the UAE, and called on people to be reassured of the vaccination.
BIBP’s COVID-19 vaccine was approved by the National Medical Products Administration (NMPA) for conditional marketing on December 30, 2020 in China. This vaccine was approved for registration and marketing in the UAE, Bahrain, Bolivia and Seychelles on December 9, 2020, December 13, 2020, February 5, 2021 and February 25, 2021, respectively, and has been approved for use or emergency use in more than 30 countries and regions worldwide. More than 10 heads of state and political leaders have been vaccinated with BIBP’s COVID-19 vaccine, which shows that the technical safety, efficacy, general applicability, capacity and accessibility, and convenience of transport and storage of the vaccine have been highly recognized by the international community.
BIBP will spare no effort in the R&D, production and use of COVID-19 vaccines, and continuously carry out the Phase III clinical trials, so as to provide strong support for realizing the accessibility and affordability of COVID-19 vaccines as a global public good, and contribute China’s part to the world.
