China’s COVID-19 Vaccine Obtained EU GMP Certification for the First Time

On April 1, Hungary’s drug regulator issued the EU GMP certification to the Inactivated COVID-19 Vaccine (Vero Cell) developed by Beijing Institute of Biological Products Co., Ltd. (BIBP), China National Biotec Group Company Limited (CNBG), Sinopharm (BIBP’s COVID-19 Vaccine). This is the first vaccine product approved for use and granted GMP certification by the European Union in China’s history, marking a new step for China’s COVID-19 vaccine to become a global public good.

From January 13 to 15, Hungarian National Institute of Pharmacy and Nutrition (OGYÉI) conducted a GMP audit on SINOPHARM CNBG BIBP, and concluded after a strict audit and comprehensive judgment that, the vaccine met the EU standards and was approved for emergency use. On March 3, SINOPHARM CNBG BIBP submitted a follow-up report to Hungary’s drug regulator. On April 1, Hungary’s drug regulator officially issued the EU GMP certification to BIBP’s COVID-19 vaccine in accordance with the EU regulatory standards and rules.

On February 16, the first batch of BIBP’s COVID-19 vaccines was delivered to Budapest, the capital of Hungary. On February 24, Hungary began vaccination with BIBP’s COVID-19 vaccine, making it the first EU state to use a Chinese vaccine.
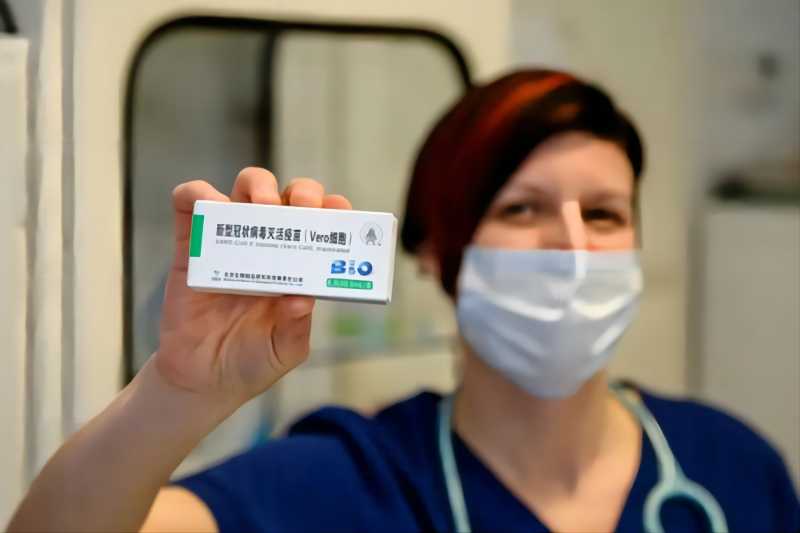
On February 24, a family doctor in Varoshred, Hungary showed a COVID-19 vaccine produced by SINOPHARM CNBG BIBP. Photo by Xinhua News Agency
On February 26, President János Áder received the first dose of BIBP’s COVID-19 vaccine. He called on the public to trust the physicians and healthcare system of Hungary, urging people to register and get the COVID-19 vaccine approved by Hungary’s drug regulator as soon as possible. On February 28, the third day after the President was vaccinated, Premier Orban announced through social media that he had also been vaccinated with BIBP’s COVID-19 vaccine in Budapest, the capital of Hungary.
BIBP’s COVID-19 Vaccine was approved for conditional marketing on December 30, 2020 in China, approved for registration and marketing in the UAE, Bahrain, Bolivia and Seychelles on December 9, 2020, December 13, 2020, February 5, 2021 and February 25, 2021, respectively, and has been put into use in 54 countries and regions worldwide. These all prove that the technical safety, efficacy, general applicability, capacity and accessibility, and convenience of transport and storage of the vaccine have been highly recognized by the international community.
Carry on the centenary legacy and keep watch for life
