BIBP’s COVID-19 Vaccine Approved for Emergency Use in Philippines
On the evening of June 7 local time, Rolando Enrique Domingo, Director-general of Food and Drug Administration (FDA) of the Philippines said that the Philippines has officially approved the Inactivated COVID-19 Vaccine (Vero Cell) developed by Beijing Institute of Biological Products Co., Ltd. (BIBP), China National Biotec Group Company Limited (CNBG), Sinopharm (hereinafter referred to as “BIBP’s COVID-19 vaccine”) for emergency use.
This progress was announced at a meeting held by President Rodrigo Duterte with the Joint Anti-COVID-19 Task Force of the Philippine government, which made BIBP’s COVID-19 Vaccine the eighth COVID-19 vaccine approved for emergency use in the Philippines.
 △
△
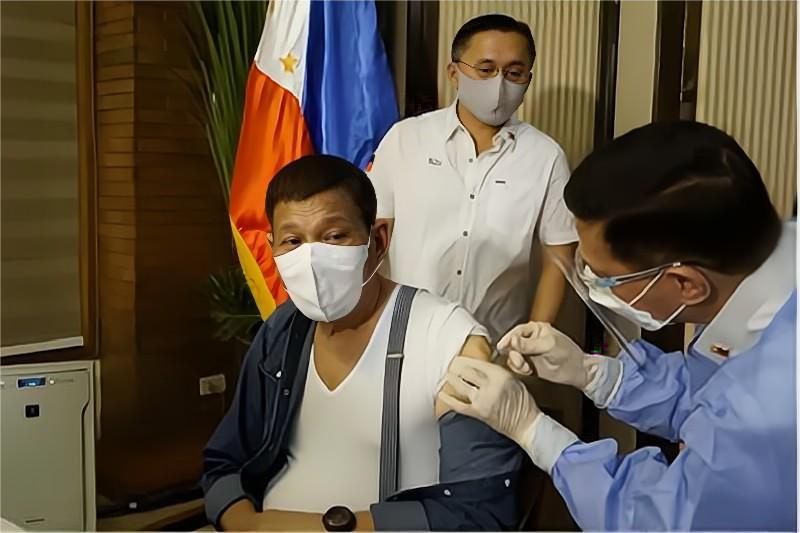
President Rodrigo Duterte Was Vaccinated with BIBP’s COVID-19 Vaccine on May 3. Photo from PCOO
