Good News | National Innovation Platform + 1
Recently, Beijing Institute of Biological Products Co., Ltd. (BIBP), a subsidiary of China National Biotec Group Company Limited which is subordinated to China National Pharmaceutical Group Co., Ltd, has been accredited as the 2023 (30th batch) National Enterprise Technology Center. This recognition reflects the acknowledgement of BIBP's high-level scientific and technological innovation and high-quality development in the new era by the industrial authorities and the industry.

The National Enterprise Technology Center is one of the highest-level and most influential technology innovation platforms jointly awarded by the National Development and Reform Commission, Ministry of Science and Technology of the People's Republic of China (PRC), Ministry of Finance of the PRC, General Administration of Customs of the PRC, and State Administration of Taxation. The accreditation process requires a comprehensive assessment of the declared enterprise's level of development in the industry, advantages, innovative management system, research and development investment, equipment and facilities, and other items. Recognized enterprises must have strong technological innovation capability in major industries of the national economy, remarkable innovation performance and the advantage of having an important demonstration role. Therefore, the evaluation results can comprehensively and objectively show the scientific and technological level of the enterprise and its ability of independent innovation.

BIBP Innovation Center
BIBP has a 105-year development history and is the cradle of the modern chinese biological products industry. As a modern high-tech company with a century-old history, it actively focuses on the needs of people's health and plays an important role in the supply of vaccines for the National Immunization Program and national public health security.

BIBP Innovation Center
In recent years, it has developed and launched the Poliomyelitis Vaccine (Vero Cell), Inactivated, Sabin Strains, the world's first COVID-19 Vaccine (Vero Cell), Inactivated, and other major products to protect people's health. Existing products Poliomyelitis (Live) Vaccine Type I Type III (Human Diploid Cell), Oral, Poliomyelitis Vaccine (Vero Cell), Inactivated, Sabin Strains and COVID-19 Vaccine (Vero Cell), Inactivated, have all been approved by World Health Organization (WHO) and got pre-qualification. BIBP has been recognized as the national high-tech enterprise, the national technological innovation demonstration enterprise, the Beijing enterprise technology center, the national postdoctoral research station, and many other qualifications and honors, and has a high industry reputation.
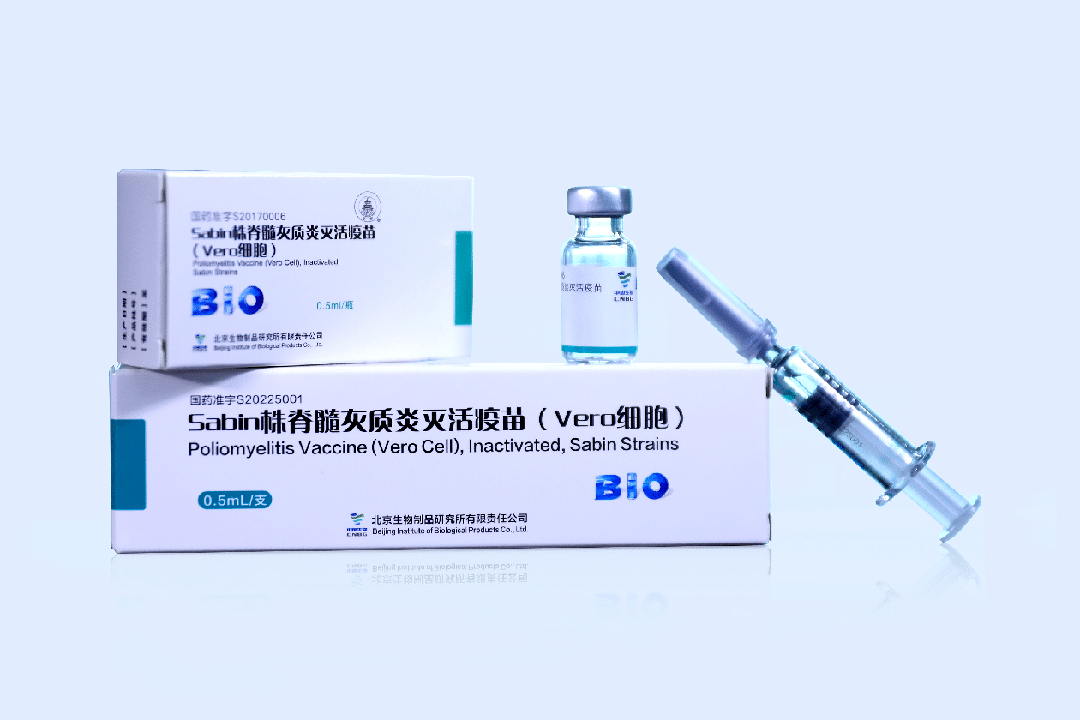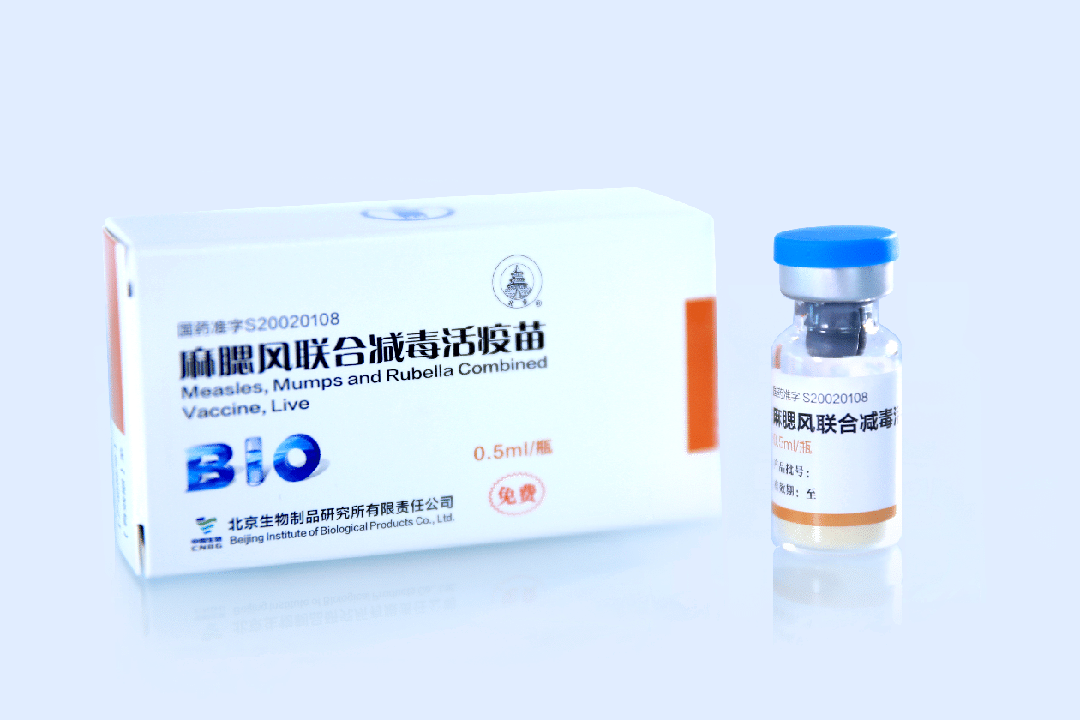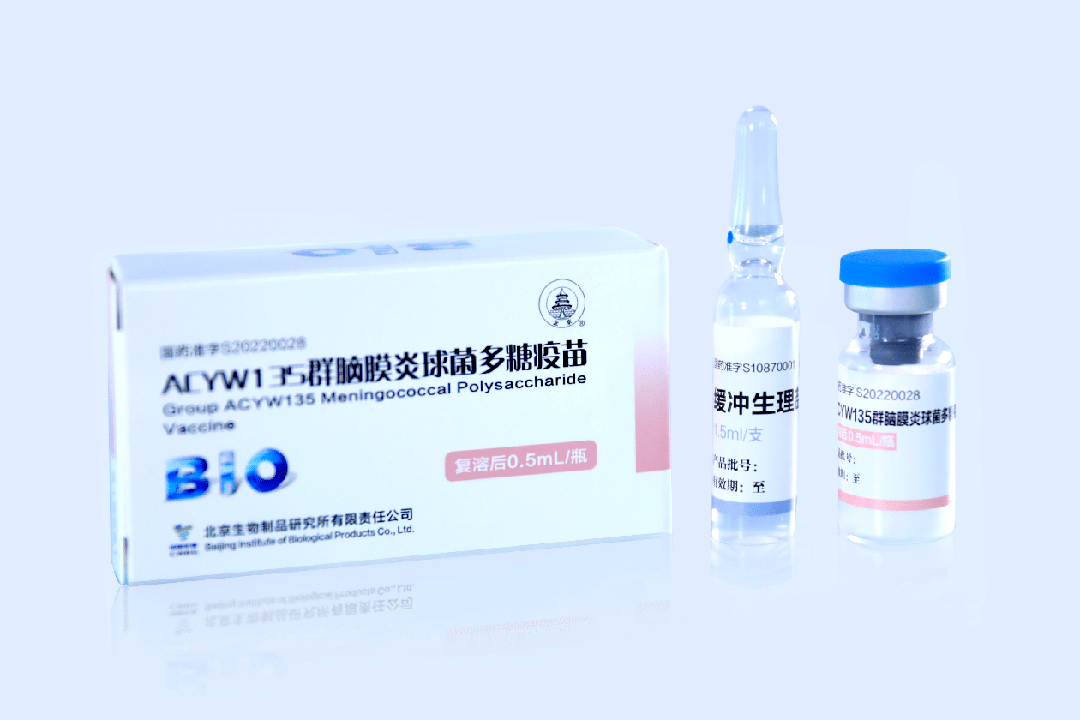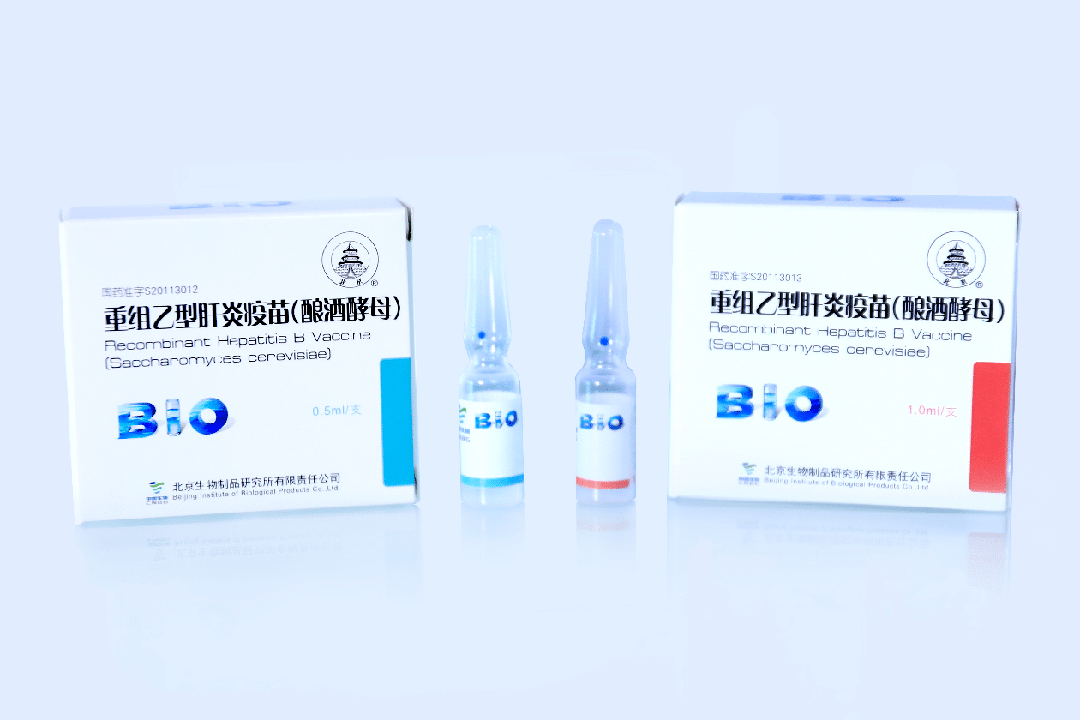
BIBP Main Product
Relying on the national enterprise technology center, BIBP will continue to adhere to the concept of innovative development, continue to increase investment in research and development, strengthen the enterprise's technological innovation system and talent team construction, and create joint vaccines, tumor drugs, gene therapy drugs and small molecule drugs technology platforms, strengthens the leading and promoting role by core business in the industry, aim at the world's technological frontier, promote the development of chinese strategic emerging industries, and better serve the country's major needs and people's lives and health.

BIBP Innovation Center
